
What is the melting point of cobalta)1449 Kb)1559 Kc)1669 Kd)1769 KCorrect answer is option D. The melting point is the temperature at which the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid.Ĭobalt – Properties Element Cobalt Atomic Number 27 Symbol Co Element Category Transition Metal Phase at STP Solid Atomic Mass 58.9332 Density at STP 8.9 Electron Configuration 3d7 4s2 Possible Oxidation States +2,3 Electron Affinity 63.7 Electronegativity 1.88 1st Ionization Energy 7.881 Year of Discovery 1735 Discoverer Brandt, Georg Thermal properties Melting Point 1495 Boiling Point 2927 Thermal Conductivity 100 Specific Heat 0.42 Heat of Fusion 16.19 Heat of Vaporization 376. Quick links for Chemical Engineering exam. At some point, the amplitude of vibration becomes so large that the atoms start to invade the space of their nearest neighbors and disturb them, and the melting process initiates. As a solid is heated, its particles vibrate more rapidly as the solid absorbs kinetic energy.

The motion of individual atoms, ions, or molecules in a solid is restricted to vibrational motion about a fixed point. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as common window glass), and are typically low in energy. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. The first theory explaining the mechanism of melting in bulk was proposed by Lindemann, who used the vibration of atoms in the crystal to explain the melting transition. It is used chiefly for magnetic alloys, high-temperature alloys, and in the form of its salts for blue glass and ceramic pigments. When considered as the temperature of the reverse change from liquid to solid, it is called the freezing point or crystallization point. The melting point of a substance depends on pressure and is usually specified at standard pressure. Adding heat will convert the solid into a liquid with no temperature change. It also contains basic descriptions and applications. at high temperatures and superalloys that are used near their melting points. In thermodynamics, the melting point defines a condition where the solid and liquid can exist in equilibrium. This article contains comparison of key thermal and atomic properties of cobalt and copper, two comparable chemical elements from the periodic table. 1735) by a Swedish chemist, Georg Brandt, though cobalt compounds had been. The alloy composition used in orthopedic implants is described in industry standard ASTM -F75: mainly cobalt, with 27 to 30 chromium, 5 to 7 molybdenum, and upper limits on other important elements such as less than 1 each of manganese and silicon, less than 0.75 iron, less than 0. When considered as the temperature of the reverse change from vapor to liquid, it is called the condensation point. The pressure at which vaporization (boiling) starts to occur for a given temperature is called the saturation pressure. The temperature at which vaporization (boiling) starts to occur for a given pressure is called the saturation temperature or boiling point. In thermodynamics, saturationdefines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. It also can be used in dental care.Note that these points are associated with the standard atmospheric pressure. It is also used in medicine, in electroplating, and in agriculture.Ĭobalt(II) fluoride, a compound with the formula CoF2, is used as a catalyst to alloy metals.

Cobalt can be used in the production of catalysts too. Cobalt had been used in imparting a blue color to glazes, glass, and ceramics. Cobalt compounds are used in the manufacture of porcelain enamels and batteries as well as in paints and inks. Cobalt blue has been used as a coloring agent in jewelry and Chinese porcelain. It also alloys with aluminum, platinum, iron, and nickel. Uses of CobaltĬobalt, a chemical element with the symbol Co, is used in making magnets. Due to its high strengths, the alloys of this metal are used in aircraft production, etc. With industrial purposes, cobalt is usually used in alloys with iron, nickel, and aluminum, to produce powerful magnets. If consumed in larger doses, cobalt is very carcinogenic. This element is crucial for all living organisms, but in micro doses. This element can be found in some minerals, as it was discovered that there are some cobalt deposits at the bottom of the oceans. This metal used to be one of the materials widely used in ancient China to produce porcelain and add a bit of beautiful blue color to it.
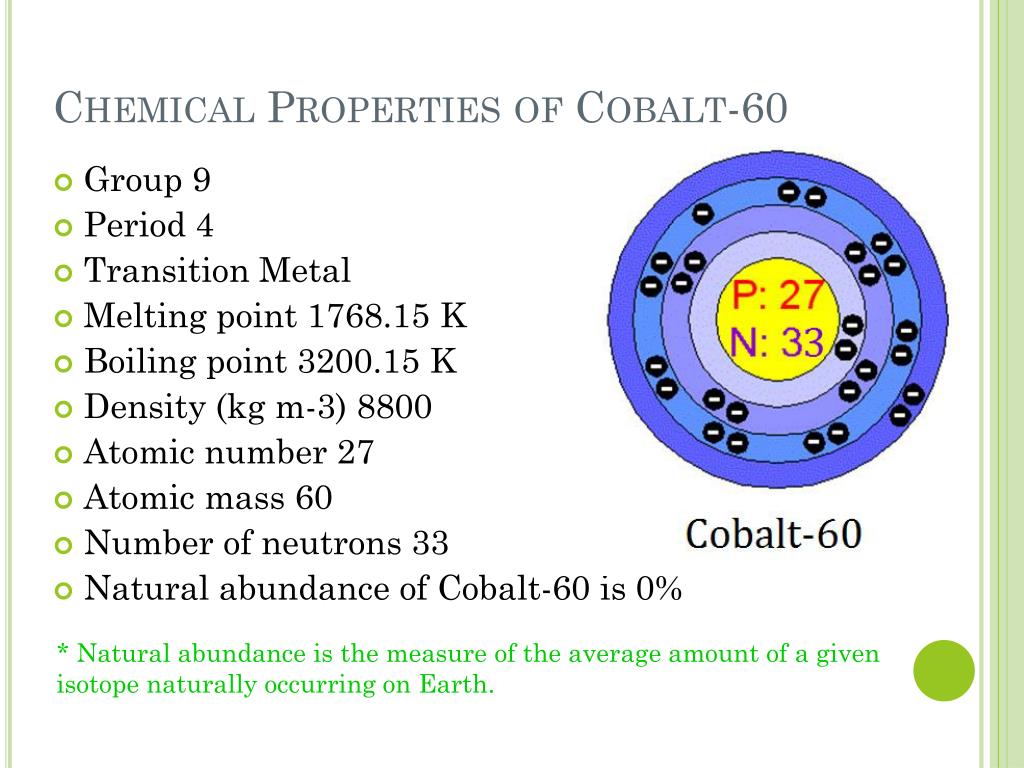
Its name is believed to take roots from a German word meaning goblin. Cobalt is a light grey and bluish metal, which is similar to iron in its qualities and can be easily magnetized.


 0 kommentar(er)
0 kommentar(er)
